Divide this text into sentences and correct mistakes: 1. Oh, are you a domestic importer of tobacco products with questions about FDA's regulations? 2. Stay tuned for some answers and tips on this edition of FDA's tobacco compliance webinars. 3. Music Applause Applause Music. 4. Welcome to FDA's tobacco compliance webinar education and information for retailers and small businesses, sponsored by the US Food and Drug Administration and the Center for Tobacco Products. 5. I'm David Erasing. Thanks for joining us today. 6. We received many questions from domestic importers of tobacco products about FDA's regulations. 7. As we've done in the past, we'll be answering some of those questions today and giving importers some additional tips on how to comply with the regulations. 8. Joining me today is my colleague, Christina Peters, from CTP's Office of Compliance and Enforcement. 9. You'll notice that some of the slides have links to relevant guidance documents, letters, and past webinars that can be found on FDA's website. 10. We encourage you to review those materials in addition to today's webinar. 11. Now, let's get started. 12. Commander Peters, thanks Captain Racine. 13. The purpose of this webinar is to answer some questions we are frequently asked by importers of tobacco products and to provide some tips that we think may be helpful to importers. 14. For importers who have not seen our 2016 imports webinar, we strongly encourage you to watch that webinar as well. 15. Although some of the deadlines for meeting certain requirements have changed since that video was made available, the topics we will be discussing today include an update on the dates to meet certain requirements, which have been revised. 16. The automated commercial environment, also known as ACE, and which data elements are and are not required when...
Award-winning PDF software





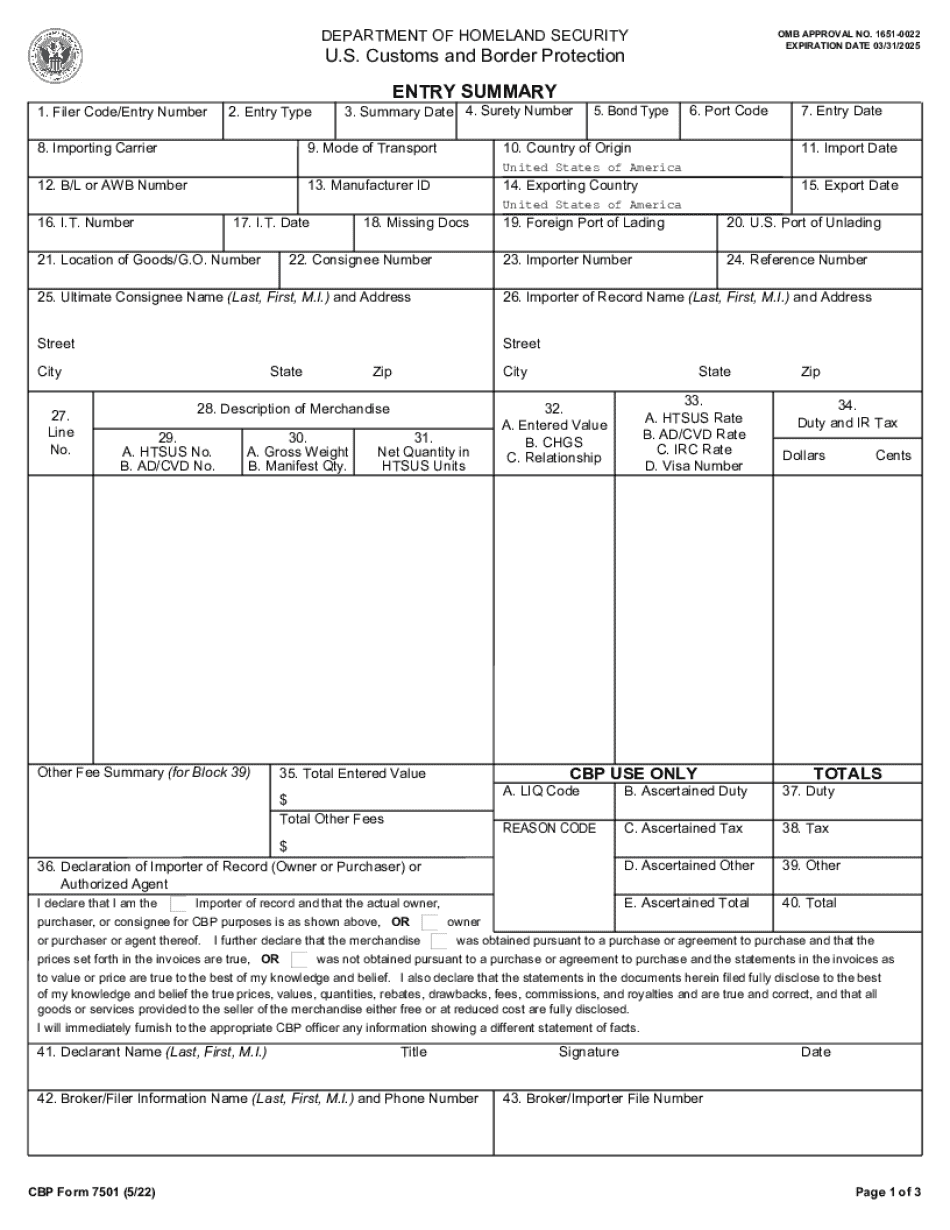
19 cfr importer of record Form: What You Should Know
S. Mailing address as the address from which services and any applicable fees may be paid or provided. An MIN number shall be the same number as that on the identification (MIN) card of the individual and all correspondence received should be routed to an MIN that is identical in form to the number on the individual's MIN card or by an MIN that includes the individual's MIN status as part of its electronic or physical description. An MIN number is issued by 19 CFR § 24.5. — Filing identification number. — Federal Register No person, firm, or agency involved in the importation of the goods in question need need to provide a social security number to the importer. The importer is responsible for completing the information required on Form 5106 and filling in all appropriate fields. An 19 CFR § 24.5. — Immediate action in certain cases when the identity of the importer is unknown — eCFR If you are not the importer listed on the document described above but are the consignee, you should be filed in block 6 of the form. Form 5106 is a required document to be filed by parties to the importation when they are unable to identify themselves before Customs. 19 CFR § 24.5. — Failure to submit identification number or other verification. — Federal Register An importer's identity information will be withheld if the individual has no United States address. Exporters may complete a customs entry summary (CES). CES Form 743 (MIN 4-10-2014 for Importers) is required to report information to CBP that identifies an importer. Example: An importer's MIN is (1) and there are no United States addresses listed. The Form 743 must be filed.
online solutions help you to manage your record administration along with raise the efficiency of the workflows. Stick to the fast guide to do Cbp Form 7501 Instructions, steer clear of blunders along with furnish it in a timely manner:
How to complete any Cbp Form 7501 Instructions online: - On the site with all the document, click on Begin immediately along with complete for the editor.
- Use your indications to submit established track record areas.
- Add your own info and speak to data.
- Make sure that you enter correct details and numbers throughout suitable areas.
- Very carefully confirm the content of the form as well as grammar along with punctuational.
- Navigate to Support area when you have questions or perhaps handle our assistance team.
- Place an electronic digital unique in your Cbp Form 7501 Instructions by using Sign Device.
- After the form is fully gone, media Completed.
- Deliver the particular prepared document by way of electronic mail or facsimile, art print it out or perhaps reduce the gadget.
PDF editor permits you to help make changes to your Cbp Form 7501 Instructions from the internet connected gadget, personalize it based on your requirements, indicator this in electronic format and also disperse differently.
Video instructions and help with filling out and completing 19 cfr importer of record